Archive for the ‘Diseases’ Category
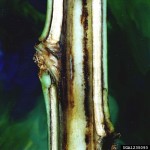
Bacterial Wilt (Granville Wilt)
Varieties Resistant to Bacterial Wilt:
Flue Cured: CC 67, CC 37, SP 236, SP 227
Burley: CC 812G
Pictures provided by R.J. Reynolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, Bugwood.org and Clemson University – USDA Cooperative Extension Slide Series, Bugwood.org
Granville wilt is most damaging in fields where tobacco was grown the previous year, in wet areas in a field, and in years where soil temperatures are normal to above normal. Other plants the bacteria can infect include tomatoes, Irish potatoes, pepper, eggplant, peanuts, and weeds.
The first symptom is a wilting on one side of the plant. As the disease progresses, the entire plant wilts and death generally follows. When death does not occur, plants are usually stunted and leaves may be twisted and otherwise distorted. The stalk usually turns black, especially at the ground level. At this stage, Granville wilt may be easily confused with other diseases such as black shank. Dark streaks can be seen extending up the plant just beneath the outer bark. Infection may not be noticed immediately because wilting symptoms may not appear until the plant undergoes moisture stress. It is not unusual to observe symptoms several weeks after initial infection. A simple diagnostic test for Granville wilt can be done on-farm. When an infected stem segment is suspended in a glass of clear water for a few minutes, bacterial streaming occurs. The bacterial streaming appears as white ooze or a smokey stream, which originates from the cut end of the stem, where the dark streaks are observed under the bark, and slowly moves out into the water.
Granville wilt is caused by microscopic bacteria (Ralstonia solanacearum). The bacterium is spread by anything that moves infested soil or water. Major means of spread include water, infected transplants and farm vehicles moving from field to field. The motile bacteria gain entry into the plant through natural openings or wounds. Since roots often “wound themselves” as they grow, or are wounded during transplanting, the Granville wilt bacteria have no difficulty in gaining entry into the plant. More extensive root wounding caused by nematodes or root pruning during cultivation provide more paths of entry for the bacteria. Increased disease levels in the resistant varieties have been noted where root-knot nematodes are present. High populations (greater than 250,000 bacteria per gm of soil) are usually necessary for infection to occur. The bacteria may also be spread during mechanical topping and harvesting. These bacteria are favored by relatively high soil temperatures and adequate to high moisture levels in the soil. Poor soil drainage and wet, warm growing seasons favor Granville wilt.
Crop Rotation – Crop rotation must be the basis on which Granville wilt management programs are established. This practice is perhaps the most essential thing that growers can do to minimize losses due to Granville wilt. In fact, without appropriate crop rotation it is not possible to manage this disease successfully where the infestation level is moderate to high. Therefore, crop rotation must be the basis on which Granville wilt management programs are established. Crop rotation is effective because the Granville wilt bacteria live in the soil and are not well adapted to survival in the absence of susceptible plant tissue. Thus, their populations decline if a suitable plant such as tobacco is absent for even one year. As is true with any other soil-borne pathogen, the longer the rotation, the more efficient the control. However, planting a non-host crop (soybeans, fescue, corn, cotton, milo) just one year will usually significantly reduce the disease loss in the following tobacco crop. Integrating other management practices, such as improved drainage, avoiding late or deep cultivations, stalk and root destruction, and the use of multi-purpose fumigants where disease occurred in past years, is better than relying on any one or two practices.
Stalk and Root Destruction (R-9-P) – Roots and stalks from the previous crop should be destroyed as soon as possible after harvest. The decay of old plant residue through stalk and root destruction soon after harvest decreases the number of bacteria present in the soil.
Resistant Varieties – Varieties are available which carry varying levels of resistance to Granville wilt. None of these varieties is immune to this disease and some losses might be expected in severely infested areas with the use of any variety. Nevertheless, growers have an opportunity to select those varieties which will afford them, in most cases, good protection when used in combination with other disease control practices such as stalk and root destruction and crop rotation. Consult the most recent issue of the Tobacco Information bulletin, available in local county extension centers, for resistance ratings of currently available varieties.
Chemical Control – The fumigants Chlor-O-Pic 100, Telone C-17, and Terr-O-Gas 67 may help control Granville wilt if used in combination with other cultural control practices. All require a 3-week waiting period between time of application and transplanting. Always read and follow label instructions. Consult the most recent issue of the Flue-Cured Tobacco Information, available in local county extension centers, for more information.
Black Shank
Varieties Resistant to Bacterial Wilt: CC 27, CC 37, CC 67, CC 143, NC 299, SP 227, SP 236

Click for larger |

Click for larger |

Click for larger |
Pictures provided by Clemson University – USDA Cooperative Extension Slide Series, Bugwood.org
Black shank is among the most destructive and widespread of all tobacco diseases. This disease is most prevalent in poorly-drained areas where tobacco was planted the previous year. Black shank is a warm-weather disease, which is favored by temperatures ranging from about 84 to 90 F. Once soil in a field becomes infested with the black shank fungus, it cannot be eliminated, therefore, the disease must be managed every year on a continuing basis.
The disease is characterized by a rapid yellowing and wilting followed by death of the entire plant. A dark brown to black, somewhat sunken, lesion usually appears on the stalk at or near the ground level. This lesion often extends up the stalk or shank of the plant causing it to turn black. Stalks, when split, usually reveal the blackened pith separated into discrete disks. This feature is of diagnostic value only when used in conjunction with other observations because disking may occur due to other factors. Infected plants may be scattered or uniformly distributed in a given field. Roots and crowns are usually decayed. Only root and crown symptoms may be observed in very dry years or on resistant varieties.
Black shank is caused by the fungus Phytophthora parasitica var. nicotiana which lives in the soil. This pathogen belongs to a group of fungi that occurs commonly in areas of high soil moisture. The fungus produces microscopic spores which swim in water surrounding roots and/or soil particles. These swimming spores are attracted to tobacco, their only natural host, by root exudates produced primarily at growing points and wounds. Whereas wounds are not required for penetration, they do favor more rapid disease build-up.
When the soil environment is not suitable for protection and survival of the motile spores, the fungus forms thick-walled, resistant spores some of which may survive for years during conditions unfavorable for the fungus. Once conditions are favorable, the resistant spores germinate and motile spores are produced. During favorable conditions for the fungus, a new generation of motile spores is produced every 72 hours.
The black shank fungus is spread when infested soil is moved from one place to another. Contaminated irrigation or runoff water may also aid in its movement within a field or from one field to another.
Although roots are the most commonly affected plant parts, occasionally the fungus infects leaves and forms circular, yellowish-to-brown lesions up to 3 inches in diameter. Leaf infection may occur as a result of zoospores in splashing water or contact with infested soil.
There are two known types of black shank, race 0 and race 1, and they are easily confused.
Crop Rotation – Rotation should be the foundation of any black shank management program because the fungus attacks only tobacco. Leaving the field out of tobacco for one or more years will reduce, but not eliminate, this fungus. Any crop can be grown between tobacco crops to reduce the population level of the pathogen.
Resistant Varieties – Varieties possessing various levels of resistance to black shank are available; however, they should be used as part of an integrated approach including crop rotation and other appropriate cultural practices. The latest issue of the Flue-Cured Tobacco Information bulletin lists varieties and their resistance levels. This bulletin is available in all county extension centers.
Chemical Control – Several soil-applied chemicals are labeled for black shank control. Included are the multi-purpose fumigants Telone C-17, Chlor-O-Pic 100, and Terr-O-Gas 67. However, the systemic fungicides Ridomil Gold or Ultraflourish are the most effective materials against black shank. Additional information on chemical control can be obtained from the most recent Flue-Cured Tobacco Information bulletin.
Drainage – Improving drainage in poorly drained areas reduces black shank losses by making the soil environment less favorable for infection.
Sanitation – Elimination of the host by destroying stalks and roots immediately following harvest will help reduce populations of the fungus and nematodes, resulting in less damage in the next crop.
Nematode Control – Control of root-knot nematodes helps to ensure the effectiveness of resistant varieties and fungicides. In fields infested with both the black shank fungus and root-knot nematodes, the effectiveness of black shank resistant varieties and fungicides is reduced if the nematodes are not controlled.
Cucumber Mosaic Virus
There are no cultivars resistant to CMV.
Click for larger image
R. J. Renyolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, Bugwood.org
The cucumber mosaic virus (CMV) is present all over the world but is more severe in Asian and European countries. The most common symptom is a typical mosaic that can be confused with TMV.
CMV has a large number of host plants and is transmitted by over 60 species of tobacco aphids. It is very common in areas where vegetables are produced, especially cucumbers, cucurbitaceae and solanaceae. Normally, the symptoms first appear at tobacco field borders, close to weeds and trees, the preferred hosts of insects and the virus.
As it is transmitted by aphids, a good control of this insect is critical to avoid losses to this virus disease.
There are no cultivars resistant to CMV. The disease should be prevented with systemic insecticides which have long residual effect and other measures like barrier crops that form a physical barrier between the tobacco field and potential sources of the disease. Planting close to vegetables should be avoided.
Erwinia
There are no cultivars resistant to this disease.
Pictures provided by R.J. Reynolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, Bugwood.org
Hollow stalk caused by Erwinia Carotovora, is soft rot that normally affects plants in the final stage of crop cultivation. Wounds and high humidity are normally the causes. Although the bacteria can enter the stalk through any wound or stem lesion, the wounds caused at topping and the break at the top are the main entry routes. In the leaf, the bacteria may enter at the base and then extend to the midrib, but they most commonly reach the pith, which is rapidly destroyed by a soft rot, and the stem becomes hollow. In the outer side of the plant the lesions appear in the form of dark streaks along the stem. Once the pith has died, the leaves wilt and turn yellow, and sometimes fall from the stalk.
The bacteria survive for years and they occur naturally in the soil and even on leaves of any plant, facilitating infection through wounds caused at topping and at sucker elimination. As dissemination of the bacteria is favored by humidity, so topping and sucker elimination should be avoided in cloudy, wet or rainy days. During topping, care should be taken not to touch the soil or clean the hands with soil/sand. Rainy weather increases the development of the disease.
There are no cultivars resistant to this disease, a fact that calls for preventive measures.
Etch (Tobacco Etch Virus [TEV] )
Recommended resistant varieties: NC 291
Pictures provided by R.J. Reynolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, Bugwood.org and Clemson University – USDA Cooperative Extension Slide Series, Bugwood.org
Tobacco etch virus (TEV) infects tomatoes and peppers along with other plants in the Solanaceae family.
Leaves of TEV infected plants are severely mottled, puckered, and wrinkled, and plants infected at an early age are severely stunted. The younger plants are at time of infection, the greater the reduction in yield.
The occurrence of TEV in tomato fields is closely associated with other infected solanaceous crops, especially pepper, and natural weed hosts, which serve as virus reservoirs. Thistle, lamb’s quarter, sickle pod, jimsonweed, and black nightshade, among others, can act as alternate hosts for TEV. The virus can be transmitted by at least 10 species of aphid in the non-persistent manner, meaning the aphids need to feed on a TEV-infected plant for only a few seconds to pick up the virus. There are no reports of seed transmission in any host plant.
TEV can be controlled by the following strategies. Eradicate all biennial and perennial weeds and wild reservoir hosts in and around fields. Maintain a distance of at least 30 yards between susceptible crops and weeds or other susceptible plants, including those in ditch banks, hedge or fence rows, and other locations. Plant late settings as far as possible from fields used to produce early tomatoes and peppers. These areas can act as sources of viruses and aphids for subsequent crops. Scout fields for the first occurrence of virus disease. Where feasible, pull up and destroy infected plants but only after spraying them thoroughly with an insecticide to kill any insects they may be harboring. Lastly, monitor aphid populations early in the season and apply insecticide treatments when needed.
Fusarium Wilt
Varieties with good resistance to Fusarium Wilt: SP 236
Pictures provided by R.J. Reynolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, Bugwood.org and Clemson University – USDA Cooperative Extension Slide Series, Bugwood.org
Symptoms of Fusarium Wilt typically begin on one side of the plant. The leaves on the affected side begin to wilt and may begin to yellow. Caused by a soil-borne fungus, this disease causes the leaves to slowly yellow and dry out. On the affected side of the plant, there is a uniform brown discoloration of the entire woody tissue (vascular tissue). This brown discoloration of Fusarium Wilt is in contrast with the black streaking (lines) of Bacterial (Granville) Wilt. The unilateral appearance of the symptoms and presence of vascular discoloration can cause confusion between Fusarium Wilt and Bacterial Wilt. However, the presence of bacterial streaking lines described for bacterial wilt is a reliable way to distinguish between these two diseases. With Fusarium Wilt, some roots on the affected side simply die. Fusarium Wilt, like Bacterial Wilt, is usually accompanied by root knot nematode infestations.
Fusarium Wilt can survive in the soil for 10 years or more. It is a parasite that enters roots through wounds caused by nematodes or other root damages, reaches the plant’s vascular system and spreads through the vessels, partially blocking them and reducing the plant’s water supplies.
The fungus may be spread to other fields by vegetables or weeds. To avoid Fusarium Wilt infestations, the use of resistant cultivars is recommended, but resistance is not complete. The use of nematicides and crop rotation are also necessary and beneficial for areas seriously infected with nematodes and Fusarium.
Nematodes
Recommended Varieties for Nematodes: CC13, CC 33 and CC 35
Pictures provided by Clemson University – USDA Cooperative Extension Slide Series, bugwood.org and R.J. Reynolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, bugwood.org
Nematodes are microscopic round worms. Several types of nematodes attack tobacco plants. The most severe are the root knot nematodes, which feed on the roots. These nematodes stunt the plants and may cause the leaves to turn pale, irregular plant growth, excessive wilting by day, and poor response to fertilizers.
Nematode problems generally occur in specific areas within a field which are deficient in nitrogen and potassium. Plants in these areas may show signs of leaf tip burn. In some spots, plants may show severe symptoms, while other spots show no signs of any attack. A detailed analysis of root and soil samples is needed to correctly diagnose the problem. Nematode problems occur more frequently in warm climates with sandy soils. High temperatures accelerate the life cycle of nematodes.
There are several cultivars resistant to Meloidogyne incognita, races 1 and 3; however, damages caused by other types have been rising year after year. Besides the M. incognita, the most common types are M. javanica and M. arenaria. Cross Creeks Seed owns the largest tobacco dedicated nematode nursery in the world. Cross Creek Seed’s breeding program develops hybrid varieties, which, in addition to their resistance to types 1 and 3 of the M. incognita group, are also tolerant to M. javanica, M. arenari, and Cyst.
All Virginia hybrids released by Cross Creek Seed are resistant to M. incognita and the ones listed below are also resistant to M. javanica and M. arenaria types.
Potato Virus (PVY)
Recommended resistant varieties: NC 291, CC 55
Pictures provided by R.J. Reynolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, bugwood.org
An infection of PVY is initially indicated by a whitening of the veins of the leaves. Later, the leaves dark green stripes along the veins associated with light green leaf tissue surrounding the veins. This is commonly referred to as vein banding. This condition is often associated with the development of a light mottled color in the remaining leaf tissue. More severe types of PVY result in the necrosis of the veins of the infected plants.
Systemic necrosis often plagues cultivars which are resistant to root knot nematodes. As the lower leaves ripen, the veins on these leaves turn black. The death of the plant occurs shortly after and the leaves begin to fall off the stalk. Additionally, the stalk may split longitudinally and areas of necrosis indicated by black discoloration may be seen from the bottom to the top of the stalk. PVY resistant varieties which become infected in early stages of development my show weak symptoms without the death of the plant.
This virus is transmitted from vegetables and weeds to tobacco by migrating aphids. Plant beds or fields near potato crops, vegetables or weed infested areas should be avoided.
The use of varieties which are PVY resistant is the most effect way to prevent loss cause by PVY damage. However, it is still very important to ally insecticides for controlling aphids and other insects, which transmit the PVY virus as well as other diseases.
Tobacco Mosaic Virus (TMV)
Recommended resistant cultivars are: CC 27, CC 37, CC 67, and CC 400
Pictures provided by R.J. Reynolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, Bugwood.org and Clemson University – USDA Cooperative Extension Slide Series, Bugwood.org
Symptoms of Tobacco Mosaic Virus generally first appear as light green coloration between the veins of young leaves, followed by a “mottled” appearance of the leaf (alternate areas of light and dark green tissue). The plant may develop rough tissue which will burn the high heat associated with hot, sunny days. Symptoms on seedlings are generally much milder and are therefore easily overlooked. Stunting and mild mottling may be observed. As symptoms may be mistaken for other virus diseases like PVY or CMV, the first step in controlling mosaic is to be sure that the virus causing the mosaic symptoms is indeed TMV.
Tobacco mosaic virus is transmitted mechanically by any means that results in the virus coming in contact with injured cells of a host plant. The primary mechanism for this is contaminated worker’s hands or equipment that comes into contact with a healthy plant. For this reason there my be a larger number of TMV-infected plants in some rows, where transmission occurred during planting or cultivation practices. Contaminated hands can be freed of the virus by washing with a detergent. The virus can be inactivated on equipment by scrubbing it with a brush using detergent or by steaming. Although the virus is transmitted primarily on worker’s hands and equipment, anything that mechanically moves the virus from a source to a healthy plant can transmit it. Chewing insects, such as flea beetles and grasshoppers, are capable of transmitting the virus, but such transmission is very rare in nature. Seed may be infested with the virus, but seed transmission has not been proven.
The virus can live in the soil for long periods of time, normally in the roots, crop remains or other host plants. After infection, there is no cure or remedy against this virus disease, which prompts the need for preventive measures. The best technique to avoid TMV infestations is the use of resistant cultivars.
In the case of susceptible cultivars, when there are small numbers of infected plants in the field, the infected plants should be removed in order to prevent the disease from spreading. When growers have an infected field, all cultivation practices should always start in the healthy fields, and then progress to the infected areas. Equipment should be thoroughly cleaned after leaving infected areas.
Tomato Spotted Wilt Virus (TSWV)
There are no cultivars resistant to this disease.
Pictures provided by William C Nesmith, University of Kentucky Research and Education Center, bugwood.org, Robert M. McPherson, University of Georgia, bugwood.org and J. Michael Moore, University of Georgia, bugwood.org
Symptoms of spotted wilt vary according to the age of the plant at the moment of infection, environmental conditions and the part of the plant that is infected. Infections can occur from seedling stage through maturity. Tomato Spotted Wilt Virus may stunt the growth of infected plants. The symptoms are normally more severe on one side of the infected plants and in the apical bud, which may be distorted and bend in one direction or another. Young leaves show yellowish spots which can turn into necrotic spots of reddish-brown color. Tissue necrosis along midribs is also a common characteristic of spotted wilt. When infections occur close to flowering, ring spots may develop along the stem, with dark streaks on one side of the plant.
Spotted wilt is caused by the TSWV virus, which is transmitted by very small insects, thrips.
Damages can be considerable, and may cause the plant to die. The later the infection takes place, the smaller the damage. If infections occur close to, or at flowering, there will be small losses.
There are no cultivars resistant to this disease and, therefore, preventive measures should be taken, particularly in previously affected areas.
To prevent the disease, plants that host thrips should be eliminated from areas close to seedbeds, greenhouses and tobacco fields. Thrips can survive in several weeds, especially in broad leaf species. The application of preventive insecticides to control the insect from seedbed to field is of fundamental importance.